Over the past few months, it is possible that some of your customers have no longer been able to use the International Material Data System (IMDS) and have instead asked you to submit data using IMDS’s sister tool, Compliance Data Exchange (CDX). While IMDS is specifically intended for regulatory compliance in the automotive sector (unless special permission is granted in some rare cases for non-automotive components), CDX is meant to be used for all other non-automotive sectors.
While both platforms present a very similar look and feel, CDX comes with a few specific differences, including its own set of powerful functions that can be leveraged to speed up your regulatory reporting and increase quality controls. Both interfaces are also able to interact via the powerful optional IMDS AI tool, further increasing the quality and efficiency of your reporting.
If you need to transition from IMDS to CDX, here are a few key takeaways to help you hit the ground running.
CDX License Models
The first thing to understand is what tier of CDX you will need and whether or not you will need to pay for it.
While IMDS is a free tool made by a consortium of automotive original equipment manufacturers (OEMs), CDX is only free of charge when used for basic functions. The free version includes the ability to evaluate Material Data Sheets (MDSs) against a wide-ranging list of regulations before submitting them to your customers.
CDX Paid Licenses
If you are planning on accepting data from suppliers, you will have to select one of the several available CDX licenses. Tiers are priced differently based on your number of suppliers.
You can view the different tiers on the CDX website to determine which CDX license your company might need.
IMDS AI License
If you have been using IMDS for years and some of your parts and assemblies are used both in the automotive and non-automotive sectors, you can choose to purchase the IMDS AI License. This allows you to import your existing MDSs from IMDS to CDX.
It is important to note that once you import a datasheet submitted by one of your IMDS suppliers, the initial import will mention the source of the IMDS datasheet. However, once you make changes to it in CDX, the datasheet becomes your company’s responsibility. In this case, you might want to ask your suppliers to submit their MDS to CDX directly, with the understanding that this will require substantial work on their end if they do not opt to use an IMDS AI License themselves.
There is a yearly cost associated with the IMDS AI License, which depends on the number of datasheets you are planning to import.
Transitioning from IMDS to CDX
While the CDX and IMDS interfaces look and feel very similar, they do present several important differences:
- In CDX, materials, semi-component, and component nodes cannot be released internally into a full MDS once they are added to an MDS.
- If you are planning on reusing nodes for other builds, you should first create separate MDSs for each item so they can be assigned a CDX ID and referenced in the CDX MDS Library. If you do not need to reuse a material, it can sometimes speed up input to instead create the material directly within the part tree.
- CDX does not allow you to rearrange and move nodes up or down a part tree. Because of this, it is a good idea to create placeholders for components you are still trying to gather data for.
- IMDS Committee-published materials (mostly metals and plates) are called standard materials in CDX. Be sure to select that checkbox within the material search screen.
- If you intend to provide a Full Material Disclosure in CDX, you will need to select the 100% declaration checkbox under the “Ingredients” tab when entering your data.
- In CDX, Applications/Exemptions are still entered under the “Ingredients” data screen under the “Scope: own company” expandable tab. The interface provides additional visuals (interactive buttons) to help you select the applicable exemptions.

Using Plastic Masterbatches in CDX
IMDS Recommendation 001 specifies that in the specific case of plastic masterbatches, it is necessary to insert materials within a top-level material to account for the exact structure of the plastic masterbatch.
Depending on the settings, IMDS will allow a material to be declared using up to 10% of a “joker”/confidential basic substance when accounting for highly confidential substances. That is provided that the basic substances included in that “joker”/confidential substance node do not contain any potentially declarable/restricted/prohibited substances.
When keying in a plastic masterbatch, often the color masterbatch itself will contain a “Pigment portion, not to declare” that is above the 10% threshold. In the example below, this color masterbatch only represents 5% of the total material. Hence, the 40% pigment portion remains below the 10% threshold at the overall material level.
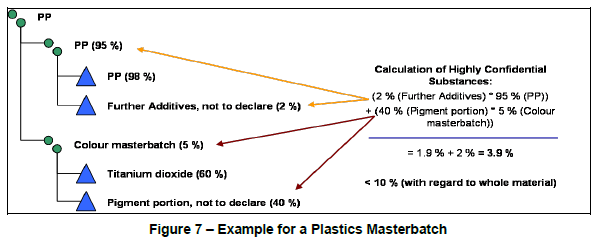
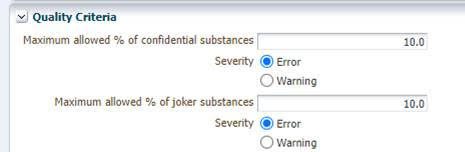
If you were to import this MDS from IMDS or manually key it into CDX, you will notice that an error appears when running a check before submitting the part to a customer. The error reads “The material contains generic substances (Joker, confidential) above the allowed maximum value of 10.0% configured by [customer name].” This will prevent you from submitting that data to your customer.
At this time, the only way to resolve this is to ask your customer to temporarily increase those two thresholds. They can do this via Administration > Company > right click on Company Name. Then click “Show” and proceed to the “Quality Criteria” section to make the necessary edits. Once this is done, you should agree on a mutually convenient timeframe during which you will be able to release the entry internally and propose it before it is changed back.
CDX Export Capabilities for Third-Party Tools
One notable key functionality offered by CDX is the ability to export IPC1752A XML documents that can then be forwarded to your customers for use with third-party regulatory compliance tools. This can save you significant time and resources, avoiding extensive manual entry that would otherwise be needed to key in your full materials declaration (FMD) into proprietary third-party tools.
Get CDX Support
CDX is a powerful tool that can help you across the compliance world. While it comes with a small learning curve, experience with IMDS can help you achieve consistent compliance with CDX.
If you need help setting your company up to effectively use CDX, training your employees in CDX, or understanding how CDX can help you meet your regulatory obligations and customer requirements, contact Tetra Tech’s CDX experts today. We can help you harness the power of CDX to reach new heights in regulatory compliance.






